

Therefore, we propose that UCHL1 is an integrative regulator of glucose metabolism, mitochondrial homeostasis, and PD pathogenesis. Our metabolic analysis identify that specific glycolytic metabolites are decreased and an energy-dependent mitophagy pathway is induced by inhibition of UCHL1. In the present study, we find that the loss of UCHL1 regulates glucose metabolism and rescues the defects related to PD. Despite these epidemiological findings, whether the mutations in UCHL1 are directly related to PD is still highly controversial. On the contrary, several statistical studies proposed reduced incidence of PD by the S18Y polymorphism in certain populations ( 25– 29). After the initial finding, the E7A substitution of UCHL1 was associated with motor neuron dysfunctions similar to PD ( 24). Unexpectedly, one German family suffering from familial PD was identified carrying I93M mutation of UCHL1, which provides the first evidence to categorize UCHL1 as a PD-susceptible gene ( 21). Belonging to the UCH family of deubiquitinating enzymes (DUBs), UCHL1 hydrolyzes a glycine peptide bond at the C terminus of ubiquitin and is highly expressed in the brain, accounting for 1 to 2% of total brain proteins ( 22, 23). However, the exact role and molecular targets of UCHL1 have not been found yet. Since protein ubiquitination plays a crucial role in the PINK1-Parkin pathway, ubiquitin C-terminal hydrolase L1 ( UCHL1 also called PARK5) has also been regarded as an important PD-associated gene ( 20, 21).
Other PD-associated genes, such as PARK7 ( DJ-1) ( 16), PARK8 ( LRRK2) ( 17), PARK13 ( HTRA2/ Omi) ( 18), and PARK15 ( FBXO7) ( 19), have been also reported to be involved in this PINK1-Parkin pathway. Ubiquitination of various mitochondrial proteins by Parkin triggers the recruitment of mitophagy adaptor proteins and mitophagosomes ( 14, 15). Upon mitochondrial damages, PINK1 phosphorylates Parkin, which induces translocation of Parkin from the cytosol to the damaged mitochondria ( 13). Among the PD genes identified from genetic and genomic analyses of patients with PD, PTEN-induced kinase 1 (PINK1 also called PARK6) comprises a central signaling axis together with the E3 ligase Parkin (also called PARK2), and both of them play an important role in controlling mitophagy ( 8– 12). The damaged mitochondria should be removed by mitochondria-specific autophagy, mitophagy.
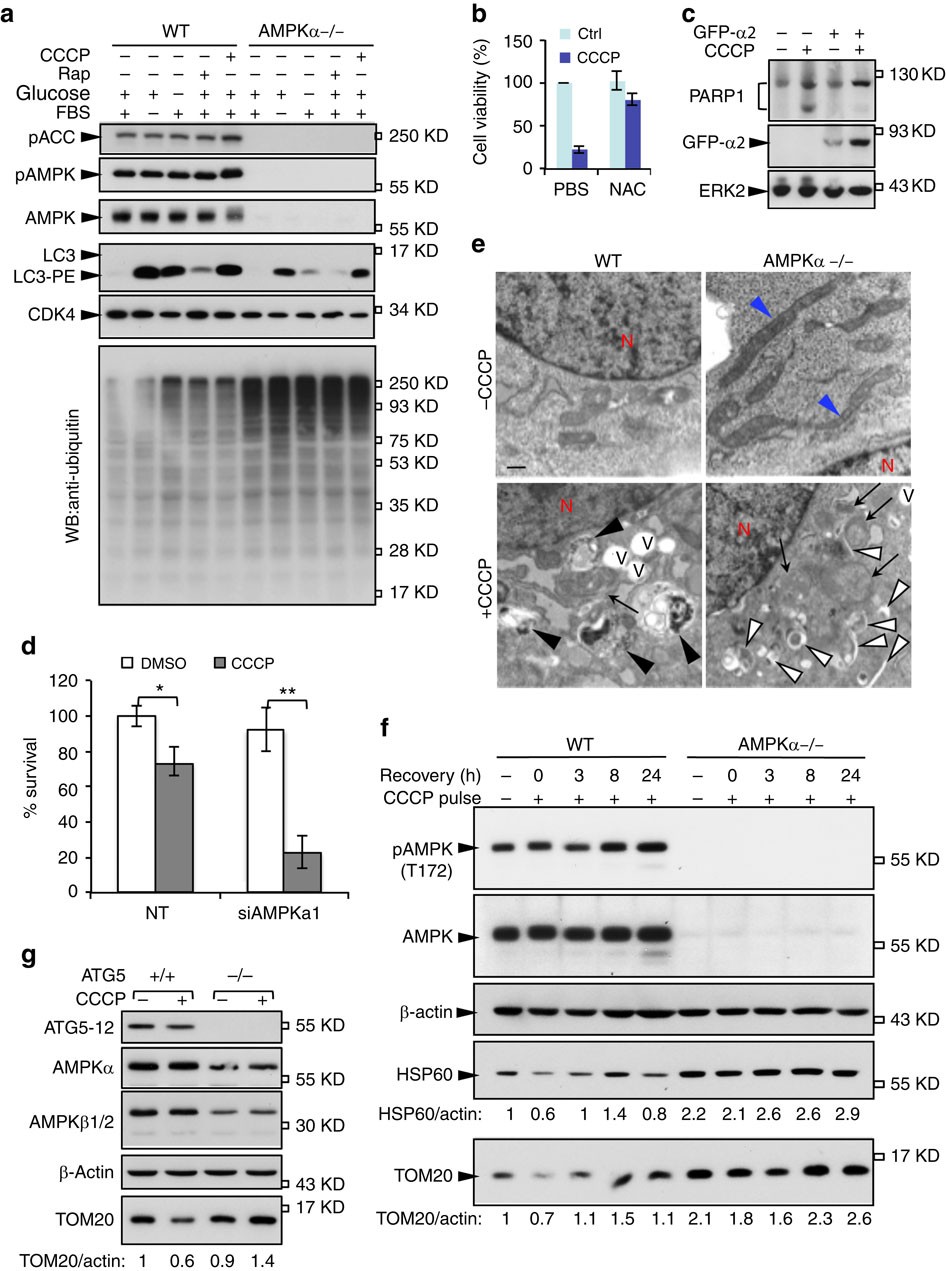
Oxidative stress and calcium accumulation are the main factors for inducing mitochondrial damages. Therefore, understanding the molecular connection between glucose metabolism and mitochondrial homeostasis has been imperative to uncover the molecular pathogenesis of PD. Consistently, inhibition of the electron transport chain (ETC) in mitochondria by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 1,1′-dimethyl-4,4′-bipyridinium (paraquat) causes degeneration of DA neurons in animal models and induces symptoms related to PD ( 4– 7). As a result, DA neurons are sensitive to oxidative stress, and the mitochondrial damages induced by ROS can be a potential cause for the death of DA neurons in patients with PD. To maintain energetic and functional demands, DA neurons are known as one of the cell types that require high-energy supplies and thus perform glucose metabolism at a high level and produce excessive reactive oxygen species (ROS) as by-products ( 2, 3). The movement disorders of patients with PD result from loss of dopaminergic (DA) neurons in the substantia nigra, a region of the midbrain ( 1). Parkinson’s disease (PD) is one of the most prevalent neurodegenerative disorders. These results suggest that UCHL1 is an integrative factor for connecting glycolysis and PD pathology. Furthermore, we identify tripartite motif–containing 63 (TRIM63) as a previously unknown E3 ligase of PKM and demonstrate its antagonistic interaction with UCHL1 to regulate PD-related pathologies. Consequently, the activated AMPK promotes the mitophagy mediated by Unc-51–like kinase 1 (ULK1) and FUN14 domain–containing 1 (FUNDC1), which underlies the effects of UCHL1 deficiency in rescuing PD-related defects. In UCHL1 knockout cells, cellular pyruvate production and ATP levels are diminished, and the activity of AMP–activated protein kinase (AMPK) is highly induced. Here, we find that the loss of UCHL1 destabilizes pyruvate kinase (PKM) and mitigates the PD-related phenotypes induced by PTEN-induced kinase 1 ( PINK1) or Parkin loss-of-function mutations in Drosophila and mammalian cells. The role of ubiquitin carboxyl-terminal hydrolase L1 ( UCHL1 also called PARK5) in the pathogenesis of Parkinson’s disease (PD) has been controversial.


 0 kommentar(er)
0 kommentar(er)
